Neurosteer Announces FDA Clearance of the Neurosteer EEG Brain Monitoring Platform
Neurosteer's EEG System Features Novel Brain Activity Representation Based on Advanced EEG Signal Processing Technology
NEW YORK, Nov. 3, 2022 /PRNewswire/ -- Neurosteer Inc. today announced the FDA clearance of its Neurosteer® single-channel EEG brain monitoring platform. This clearance allows Neurosteer's unobtrusive multi-purpose system to be used in a broad range of clinical settings. In the ICU, it can offer continuous brain monitoring to support critical interventions. In a doctor's office, it can aid in the early detection of pre-symptomatic cognitive decline, including Alzheimer's, Parkinson's and dementia. And in pharma drug trials, it can assist in the rapid and cost-effective mass screening of subjects who may be suffering from neurodegenerative disorders.
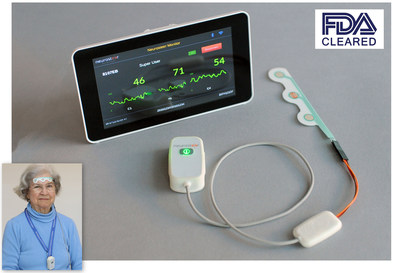
Neurosteer advances the century-old EEG into the 21st century, using an adhesive electrode strip connected to a pocket-sized sensor that wirelessly transmits EEG data to the cloud for advanced signal processing. Using just a single adhesive forehead strip, Neurosteer's innovative solution offers the traditional EEG frequency bands while also being cleared by the FDA to include several novel "brain metrics" visual representations.
These brain metrics rely on Neurosteer's advanced signal processing technology and may assist trained medical staff in making neurological diagnoses. In addition, affixing the adhesive strip to the forehead avoids interference from the hair, and using dry gel supports unattended operation for up to 12 hours. Finally, the system includes a noninvasive assessment with auditory prompts that can aid in the early detection of brain deterioration.
Dr. Amitai Bickel, Surgeon at Western Galilee Hospital, commented, "After working with the Neurosteer EEG system for several years, I've realized what an advantage it is to have a compact, easy-to-apply EEG brain monitor that can be used for real-time continuous monitoring. Neurosteer's innovative platform has the potential to revolutionize the standard of care in managing brain health."
Dr. Nathan Intrator, Founder and CEO of Neurosteer, said, "We are extremely pleased that the FDA has cleared our portable and affordable EEG system, and moreover, recognized the potential clinical value of our brain metrics visual representations. This is an important step in achieving our goal of making brain monitoring and assessment widely available to all populations."
Neurosteer Inc. has developed an innovative brain monitoring platform that is based on a portable single-channel EEG. It uses an adhesive forehead electrode strip connected to a pocket-sized sensor that wirelessly transmits EEG data to the cloud for advanced signal processing. It complements traditional frequency band analysis with "brain metrics" visual representations. The company was founded in 2015 by Dr. Nathan Intrator to commercialize brain monitoring technology that he initially developed at Tel Aviv University. With offices in New York, California and Israel, the company's patented product is currently being validated in clinical trials and used by pharmaceutical companies for patient screening and efficacy testing of new drugs.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/neurosteer-announces-fda-clearance-of-the-neurosteer-eeg-brain-monitoring-platform-301668471.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/neurosteer-announces-fda-clearance-of-the-neurosteer-eeg-brain-monitoring-platform-301668471.html
SOURCE Neurosteer Inc





Nenhum comentário